Clinical or formal audit
CPD Section 3.1 | Practice development and review
CAPE: Various, depending on the focus of the audit.
A clinical audit is a cyclical quality improvement activity that involves measurement and action. During a clinical audit, clinical practice is evaluated against a measure or standard/guideline to identify areas for improvement. The objective is to improve the quality of care and clinical outcomes. Clinical audits are a continuous process. They require ongoing monitoring and development.
A clinical audit allows you to measure whether current practice:
- meets or exceeds best practice standards, for example, RANZCP guidelines
- is relevant
- applies current knowledge
- is being effectively applied.
Examples
- How depression has been assessed and treated in a particular patient group, compared to RANZCP guidelines.
- The use and monitoring of a particular medicine, such as lithium.
- Metabolic monitoring for patients prescribed atypical antipsychotics.
- Whether letters to general practitioners meet RANZCP guidelines
- Evidence of patient education and consent being documented as part of changes to treatment.
There are many opportunities for simple and useful audits in clinical practice. See Combined Audit examples [PDF; 500KB].
How to do a clinical audit
Clinical audits have three core aspects:
Measurement: Identifying and measuring components of clinical practice.
Comparison: Comparing practice and results against recognised standards.
Evaluation: Evaluation and implementation of audit outcomes.
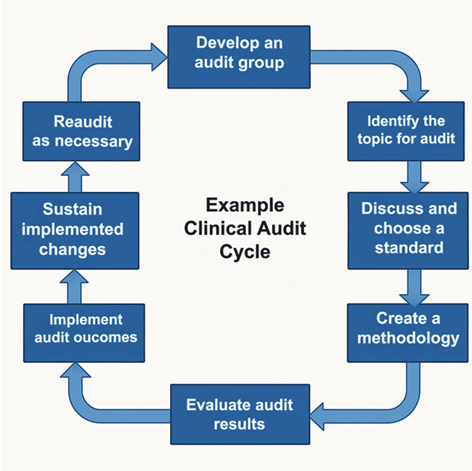
Plan Do Study Act framework
The Plan Do Study Act framework is a quality improvement tool you can use to test and implement change. Used regularly, the PDSA cycle can help improve clinical care and outcomes over time.
Following the PDSA (Plan, Do, Study, Act) framework below will ensure you meet the minimum requirements for CPD audit.
1. Plan
- Select a topic – plan a change or test around improvement goals and objectives.
- Define standards (audit criteria) to compare data against.
- Plan how and when you will collect data (methodology).
2. Do
- Collect data, document observations and problems.
3. Study
- Compare and analyse data against appropriate standards and guidelines.
- Compare results to predictions.
- Discuss possible changes and agree on what can be implemented and tested.
4. Act
- Implement agreed changes.
- Embed changes.
- Monitor the effects over time.
5. Re-audit (as necessary)
- Collect new data.
- Compare and analyse both sets of data.
- Has practice improved and/or met the required standards?
- Identify any issues.
Evidence required for MyCPD
Outline of audit including:
- aim of audit
- process, treatment or system being audited
- number of cases
- standards used
- methodology
- results
- reflection on findings
- changes to be implemented and monitored.
You may use the Clinical audit template to present this information for upload into MyCPD under Section 3.1.
CAPE domain: Various, depending on the focus of the audit.
Resources
- LearnIt module - Quality improvement module 2: Clinical audit in mental health practice
- How to do a clinical audit [PDF; 157 KB]
- RANZCP Clinical Audit template [PDF; 99KB]
- Combined Audit examples [PDF; 500KB]
- Quality Improvement Audit Project – Faculty of Psychotherapies [PDF; 25 KB]
- 101 Recipes for Audit in Psychiatry – RCPsych/OUP
